Study on fast/slow reaction mechanism of carbon-based material oxidation in high speed stream of dissociated air
-
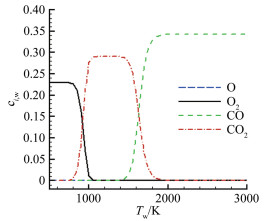
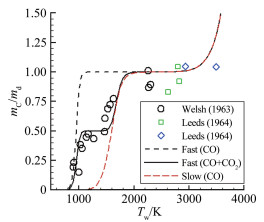
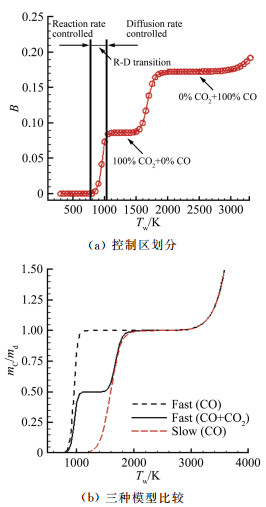
摘要: 在前期单/双平台问题研究的基础上[1],就学术上和工程应用中关注的碳在空气中燃烧的"快反应"和"慢反应"问题进行了深入分析,研究发现被广泛使用50余年的"慢反应"并不存在,而被弃置的"快反应"则真实存在,且具有重要的应用价值。采用"快反应"动力学数据,同时考虑CO、CO2两个烧蚀产物,得到的无量纲烧蚀速率随温度的变化曲线存在两个平台,其中,温度稍低情况下出现的第一平台对应的主要烧蚀产物为CO2,温度稍高情况下出现的第二平台对应的主要烧蚀产物为CO,且第一平台对应的无量纲烧蚀速率恰好是第二平台的1/2。过去常被忽略的CO2扮演了重要角色,由它产生的第一平台,将以往文献中看似完全独立、毫无关系的"快反应"和"慢反应"曲线建立了联系。理论分析表明:第一平台之前的快速上升段属于从速率控制区到扩散控制区的过渡区,第一平台及其以后的区域都属于扩散控制区(包括两个平台之间的连接线),它是由反应生成物CO与CO2的分压比δ从0到∞的变化引起的,与表面化学反应动力学条件完全无关。由"双平台"理论得到的从低温到高温、由速率控制区经由过渡区到达扩散控制区的整条烧蚀速率曲线,与实验结果完全吻合。Abstract: Based upon our previous research on the single/dual platforms theory for carbon-based material oxidation, it has been found that the generally accepted "slow" reaction model which was presented by Scala in 1962 and has been widely used till now for over fifty years does not exist, while the deserted "fast" reaction model really exists and is of great value in application. Theoretical analysis shows that the "slow" reaction appears only at the boundary-layer-diffusion-rate controlled regime, In this regime, the ablation rate has no business with the chemical reaction rates, so it is a fictitious reaction model and has no physical meanings, although the results from it may be in agreement well with test results. The products of the heterogeneous reactions of carbon with oxygen are CO and CO2, but CO2 is usually neglected when the surface temperature is higher than 1000K in many literatures. However, from theoretical analysis, author find that CO2 plays an important role in spanning the reaction range from the reaction-rate controlled regime to the diffusion controlled oxidation regime, and can't be ignored. It has been shown that for the ablation of carbon-based materials in a high speed stream of dissociated air, the "fast" reaction must be used with both products of CO2 and CO at the same time. There are two different platforms in the diffusion controlled regime. The first platform results from reactions of the predominant CO2 product, and the other is due to the predominant CO product reactions. With the increases of the surface temperature, the ratio of the mass fraction of CO to CO2 at the surface rises rapidly from zero to infinity, which causes the oxidation process to change automatically from the nominated "fast" reaction to the so called "slow" reaction lines. The dual platform theory has been confirmed by several experimental results.
-
0 引 言
风洞作为空气动力学研究的重要地面实验设备,在先进飞行器研制和基础空气动力学问题研究中发挥了不可替代的作用。实验流体力学(EFD)着眼于风洞实验数据的采集、分析和处理,是风洞系统工程中不可分割的部分。作为风洞技术测量目标之一,表面压力的测量直接关系到飞行器的升力系数与姿态控制,同时还可以通过压力分布判断飞行器表面的分离、转捩等信息。现有的压敏材料(PSP)利用非接触方式获得连续、大范围的模型表面压力分布[1],是风洞实验中有效的测压方法,然而,基于光学原理的PSP存在观测死角(如飞机短舱内表面)问题。传统的在模型表面布设测压孔的方法技术成熟度高、测量准确性好、可按需布置[2],最新发展的柔性智能蒙皮可以粘贴于模型内外表面,实现多物理量同步测量[3-4]。但测压孔和柔性传感阵列往往数量有限,需发展基于稀疏测点的场重构技术,实现壁面绕流及空间流场的感知。
流场数据具有多维度且复杂的特点,而测量值来自壁面稀疏测点,如何构建有限数据与全空间流场的映射关系是流场重构的难点所在。国内外学者从流场物理模型与数据驱动的角度进行了理论尝试,Vamsi Krishna等[5]基于快速畸变理论和Taylor假设建立双向加权模型,基于先验速度场对湍流演化进行重构。Callaham等[6]探索了基于二维流场稀疏表示和数据库的流场重构法,尝试了数据驱动的流场重构技术,并讨论了需要的随机测点最小数量。Sun等[7]结合Bayesian神经网络与先验物理模型重构了二维钟形与T形流场,并对结果不确定性进行估计。李静等[8]采用本征正交分解方法,以较少阶模态高精度再现非定常圆柱绕流的完整流场。这些重构方法大多局限在简单几何和稳态流场重构,与实际工程应用之间仍存在一定距离。得益于计算流体力学(CFD)的发展,通过数值方法求解Navier–Stokes方程[9],能够得到丰富的流场先验知识,为全流场重构提供良好的条件。然而,CFD的计算过程通常不能充分考虑风洞实际流动过程中的不确定性[10],导致确定性的计算机仿真难以复现风洞实验对物理参数的影响,从而无法实现与风洞实测数据的匹配和融合。因此,结合CFD与实测稀疏数据对风洞的真实流场进行重构极富挑战。
数据同化(Data Assimilation,DA)方法能够结合估计值和测量值进行预测 [11],可以作为联系CFD理论先验信息和EFD风洞实测数据的桥梁,其在流场重构中已有发展。Chandramouli等[12]提出一种变分数据同化方法,通过圆柱绕流在过渡状态下的三维湍流尾流案例进行方案验证,与PIV实验数据对比,验证了变分数据同化在不可压缩湍流重构中的有效性。Belligoli等[13]通过数据同化方法实现了二维翼型的迎角及马赫数的修正,降低了实验测量值与RANS模拟计算值之间的误差。集合变换卡尔曼滤波(Ensemble Transform Kalman Filter,ETKF)是卡尔曼滤波(Kalman Filter,KF)针对非线性系统模型的扩展方法[14],在复杂系统中有良好的数据同化效果。ETKF以蒙特卡洛方法为依托,以贝叶斯原理为核心,利用稀疏测量数据所包含的信息对物理场先验预报数据进行滤波,借此给出对不确定参数的连续后验估计[15]。该方法源自地球物理领域,是基于海面离散探测数据对海洋与大气的真实演化状态进行连续修正的重要工具[16]。近年来,国内外学者将该方法引入空气动力学领域,美国加州大学通过无限长薄板周围随机扰动无黏涡流模拟,印证了ETKF在流场数据同化中的有效性[17]。日本宇航研究开发机构(JAXA)应用ETKF对风洞中存在不确定性的马赫数、迎角、湍流黏度进行估计[18],成功重构刚性机翼绕流场,证实了该方法对风洞中复杂流场的估计能力。国内上海交通大学在预测直升机转子三维流场特征时,采用了ETKF优化剪切应力传输模型常数,为修正逆压梯度下边界层流动分离提供了参考[19]。
本文面向风洞实验的压力测量应用,使用集合变换卡尔曼滤波方法,以二维翼型RAE 2822和二维对称翼型NACA 0012为研究对象,进行机翼表面气动压力重构,以达到风洞实验对全域数据重构精度的要求。先通过CFD计算得到全域先验分布,结合机翼表面有限数量的压力测量值,对机翼的迎角及马赫数进行修正,重构得到高精度的机翼周围全域压力场。再采用ETKF进行流场数据同化:一方面,可以充分利用稀疏测量数据,将这些高精度数据泛化到整个流域,使得最终展示的流场更加接近真实的流场,为空间全域感知提供可能;另一方面,ETKF可以作为一种流场风洞干扰的修正方法,基于真实测量数据,直接进行流场分布的修正,获取高精度的流场分布。
1 集合变换卡尔曼滤波方法
1.1 系统模型
在流场计算中,从边界条件到物理量的分布不是简单的线性系统,而是离散的非线性系统,其中状态变量估计可通过求解Navier–Stokes方程得到。本文通过软件FLUENT对流场进行数值计算,选用Spalart–Allmaras(S–A)湍流模型。采用Navier–Stokes方程作为流动控制方程,并将此方程作为卡尔曼滤波中的系统模型:
$$ \frac{\partial }{{\partial \tau}}\iiint_V {{\boldsymbol{W}}{\rm{d}}V + \iint_S {({{{F}}_{\rm{c}}} - {{{F}}_{\rm{v}}}) \cdot {\boldsymbol{n}}}}{\rm{d}}S = 0 $$ (1) 式中:W为守恒状态矢量,包含了密度、速度和能量;V为控制体体积;Fc为对流通量;Fv为黏性通量;S为控制体表面积;n 为控制体外法线方向的单位矢量;τ为时间。
1.2 状态空间矩阵
系统模型的估计与实验测量均存在误差,卡尔曼滤波的数据同化手段通过状态空间模型将误差代入系统当中:
$$ {{\boldsymbol{x}}_t} = F({{\boldsymbol{x}}_{t - 1}}{{,\;}} {{\boldsymbol{v}}_t}) $$ (2) $$ {{\boldsymbol{y}}_t} = {\boldsymbol{H}} \cdot {{\boldsymbol{x}}_t} + {{ {ω}} _t} $$ (3) 式中:下标t表示迭代次数,${{\boldsymbol{x}}_t} $、${{\boldsymbol{y}}_t} $向量分别表示系统模型和实验测量数据的状态向量,${{\boldsymbol{v}}_t} $、${{ {ω}} _t} $向量分别表示系统模型的噪声和观测噪声,假定其符合高斯分布。非线性算子F是从第$t-1 $次迭代到第t次迭代的映射,在数据同化中,由系统模型计算得到。${\boldsymbol{H}} $矩阵是将系统估计的数据矩阵投影到实验观测数据的投影矩阵。
向量${{\boldsymbol{x}}_t} $包含了迎角α、马赫数Ma和所有(n个)计算网格节点上的密度ρ、笛卡尔速度分量u、v和压力p,其维数$l = 4n + 2$,向量${{\boldsymbol{x}}_t} $的表达式为:
$$ {{\boldsymbol{x}}_t} = {\left[ {\begin{array}{*{20}{c}} {{\rho _1}} & {{u_1}} & {{v_1}} & {{p_1}} & \cdots & {{\rho _n}} & {{u_n}} & {{v_n}} & {{p_n}} & \alpha & {Ma} \end{array}} \right]^{\rm{T}}} $$ (4) 向量${\boldsymbol{y}}_t $由实验测点对应位置的测量值构成。在稀疏压力重构中,选取实测压力p,向量${\boldsymbol{y}}_t $维数与实验测点的数量(m个)一致:
$$ {{\boldsymbol{y}}_t} = {\left[ {\begin{array}{*{20}{c}} {{p_1}}&{{p_2}}& \cdots &{{p_m}} \end{array}} \right]^{\rm{T}}} $$ (5) 1.3 集合变换卡尔曼滤波方法及实现流程
集合变换卡尔曼滤波基于最小误差方差假设,通过卡尔曼增益${\boldsymbol{K}}_t$对系统模型的估计值和实验测量值赋予权重,得到基于系统模型和实验测量值的最优状态矩阵。通过拉丁超立方抽样方法在原始边界条件一定范围内进行抽样[20],得到元素数量为k的集合,通过数值计算对集合中所有成员进行流场的预测,得到流场的先验分布矩阵${\boldsymbol{X}}_t^f$:
$$ {\boldsymbol{X}}_t^f = \left( {\begin{array}{*{20}{c}} {{\boldsymbol{x}}_t^{f(1)}}&{{\boldsymbol{x}}_t^{f(2)}}& \cdots &{{\boldsymbol{x}}_t^{f(k)}} \end{array}} \right) $$ (6) 式中:${\boldsymbol{x}}_t^{f(k)}$为通过第k组集合成员边界条件计算得到的状态向量;后文中统一用上标f表示先验分布。对得到的所有元素先验分布向量求均值得:
$$ \bar {\boldsymbol{x}}_t^f = \frac{1}{k}\sum\limits_{i\;=\;1}^k {{\boldsymbol{x}}_t^{f(i)}} $$ (7) $$ \bar {\boldsymbol{X}}_t^f = {\left[ {\begin{array}{*{20}{c}} {\bar {\boldsymbol{x}}_t^f}&{\bar {\boldsymbol{x}}_t^f}& \cdots &{\bar {\boldsymbol{x}}_t^f} \end{array}} \right]_{1 \times k}} $$ (8) 通过均值向量$\bar {\boldsymbol{x}}_t^f$求得偏差矩阵${{{\boldsymbol{X}}}'_t{^f}}$:
$$ {{\boldsymbol{X}}}'_t{^f} = \frac{1}{{\sqrt {k - 1} }}[ {{\boldsymbol{x}}_t^{f(1)} - \bar {\boldsymbol{x}}_t^f}\;\; {{\boldsymbol{x}}_t^{f(2)} - \bar {\boldsymbol{x}}_t^f}\;\; \cdots \;\; {{\boldsymbol{x}}_t^{f(k)} - \bar {\boldsymbol{x}}_t^f} ] $$ (9) 与标准卡尔曼滤波方法相同,集合变换卡尔曼滤波进行最小方差估计的关键是卡尔曼增益${\boldsymbol{K}}_t $的确定。${\boldsymbol{K}}_t $由实验观测噪声协方差矩阵$ {\boldsymbol{R}}_t $和集合成员的协方差矩阵${\boldsymbol{P}}_t^f$计算得到:
$$ {\boldsymbol{K}}_t^f = {\boldsymbol{P}}_t^f{{\boldsymbol{H}}^{\text{T}}}{({\boldsymbol{HP}}_t^f{{\boldsymbol{H}}^{\text{T}}} + {{\boldsymbol{R}}_t})^{ - 1}} $$ (10) 式中:${\boldsymbol{H}} $为式(3)中的投影矩阵,在翼型网格和实验模型的对应位置设置为1,其余位置设置为0。
$$ {\boldsymbol{H}} = {\left( {\begin{array}{*{20}{c}} {\begin{array}{*{20}{c}} 1&{} \end{array}}&\\ & \ddots &{\begin{array}{*{20}{c}} {}&1 \end{array}} \\ {\begin{array}{*{20}{c}} {}&1 \end{array}}& \end{array}} \right)_{m \times l}} $$ (11) $$ {{{\boldsymbol{P}}}_t^f}={{\boldsymbol{X}}'_t{^f}}{\left( {{{\boldsymbol{X}}}'_t{^f}} \right)^{\text{T}}} $$ (12) 实验观测噪声协方差矩阵${\boldsymbol{R}}_t $由观测噪声$ {{ {ω}} _t}$计算得到,观测噪声$ {{ {ω}} _t}$根据经验或仪器特性进行选取,为方差一定、数学期望为0的正态分布随机数${{ {ω}} _t}\propto N(0,\;{\sigma }^{2})$,反映的是对测量值的相信程度。对集合中的k组成员,对应有k个${{ {ω}} _t}$:${{ {ω}} }_{t}^{1},\;{{ {ω}} }_{t}^{2},\;\cdots ,\;{{ {ω}} }_{t}^{k}$。
$$ {\bar { {ω}} _t} = \frac{1}{k}\sum\limits_{i\;=\;1}^k {{ {ω}} _t^i} $$ (13) $$ {{ {ω}} '_t} = \frac{1}{{\sqrt {k - 1} }}\left[ {{ {ω}} _t^1 - {{\bar { {ω}} }_t}}\;\;{ { {ω}} _t^2 - {{\bar { {ω}} }_t}}\;\; \cdots \;\;{ { {ω}} _t^k - {{\bar { {ω}} }_t}} \right] $$ (14) $$ {{\boldsymbol{R}}_t} = {{ {ω}} '_t}{({{ {ω}} '_t})^{\text{T}}} $$ (15) 基于风洞实验获得的一组实验测量数据${{\boldsymbol{y}}_t}$,构建用于滤波的实验测量数据矩阵${{\boldsymbol{Y}}_t}$,矩阵${{\boldsymbol{Y}}_t}$的k列数据均取为${{\boldsymbol{y}}_t}$,以此测量数据为参考值,通过卡尔曼增益对现有先验均值矩阵$\bar {\boldsymbol{X}}_t^f$进行修正,得到后验均值矩阵$\bar {\boldsymbol{X}}_t^a$:
$$ \bar {\boldsymbol{X}}_t^a = \bar {\boldsymbol{X}}_t^f + {{\boldsymbol{K}}_t}({{\boldsymbol{Y}}_t} - {\boldsymbol{H}}\bar {\boldsymbol{X}}_t^f) $$ (16) $$ {{\boldsymbol{Y}}_t}{\text{ = }}{\left[ {\begin{array}{*{20}{c}} {{{\boldsymbol{y}}_t}}&{{{\boldsymbol{y}}_t}}& \cdots &{{{\boldsymbol{y}}_t}} \end{array}} \right]_{1 \times k}} $$ (17) 式中:上标a表示后验分布。后验偏差矩阵${{{\boldsymbol{X}}}'_t{^a}}$由协方差期望传播方程得到:
$$\begin{split} {{{\boldsymbol{X}}}'_t{^a}}{({{{\boldsymbol{X}}}'_t{^a}})^{\text{T}}} = &({{\boldsymbol{I}}_l} - {{\boldsymbol{K}}_t}{\boldsymbol{H}}){{{\boldsymbol{X}}}'_t{^f}}{({{{\boldsymbol{X}}}'_t{^f}})^{\text{T}}} \\ =& {{{\boldsymbol{X}}}'_t{^f}}{\boldsymbol{V}}{({{{\boldsymbol{X}}}'_t{^f}})^{\text{T}}} \end{split}$$ (18) $$ {\boldsymbol{V}} = {{\boldsymbol{I}}_k} + {({{{\boldsymbol{X}}}'_t{^f}})^{\text{T}}}{{\boldsymbol{H}}^{\text{T}}}{({{\boldsymbol{R}}_t})^{ - 1}}{\boldsymbol{H}}{{{\boldsymbol{X}}}'_t{^f}} $$ (19) 式中:${\boldsymbol{I}} $为单位矩阵,下标为矩阵的维度;矩阵${\boldsymbol{V}} $为对称正定矩阵。对${\boldsymbol{V}} $进行奇异值分解得到特征向量矩阵${\boldsymbol{Z}} $和特征值矩阵${\boldsymbol{G}} $,以此求得后验偏差矩阵${{\boldsymbol{X}}}'_t{^a}$:
$$ {\boldsymbol{V}} = {\boldsymbol{ZG}}{{\boldsymbol{Z}}^{\text{T}}} $$ (20) $$ {{{\boldsymbol{X}}}'_t{^a}} = {{{\boldsymbol{X}}}'_t{^f}}{\boldsymbol{Z}}{{\boldsymbol{G}}^{ - 1/2}}{{\boldsymbol{Z}}^{\text{T}}} $$ (21) 将后验均值矩阵$\bar {\boldsymbol{X}}_t^a$与后验偏差矩阵${{{\boldsymbol{X}}}'_t{^a}}$结合即可得到滤波之后的后验分布${\boldsymbol{X}}_t^a$:
$$ {\boldsymbol{X}}_t^a = \bar {\boldsymbol{X}}_t^a + \sqrt {k - 1} {{\boldsymbol{X}}}'_t{^a} $$ (22) 集合变换卡尔曼滤波的具体迭代过程如图1所示,抽样后通过FLUENT软件对集合中k组边界条件进行流场预测,在数值计算达到收敛时,导出所有网格点的密度ρ、笛卡尔速度分量u、v和压力p,构造先验分布矩阵${\boldsymbol{X}}_t^f$,通过式(7)~(15)计算卡尔曼增益${\boldsymbol{K}}_t $,然后通过式(16)~(22)计算得到此次迭代的后验分布${\boldsymbol{X}}_t^a$。迭代收敛的判断条件是数据同化前后网格点物理量的均方误差MSE降低为初次迭代的1%,此条件表明ETKF继续迭代无法对先验分布带来更大的修正效果,以网格节点处的密度ρ为例进行计算:
$$ {\rho _{{\rm{MSE}}}} = \frac{1}{n}\sum\limits_{i\;=\;1}^n {{{({\rho _i^a} - \rho _i^f)}^2}} $$ (23) 如果不满足迭代退出条件,则将后验分布中的边界条件(迎角、马赫数)重新带入FLUENT求解器中,作为下一次计算的边界条件初始值,以此获得下一次迭代的先验分布矢量${\boldsymbol{x}}_{t + 1}^f$:
$${\boldsymbol{ x}}_{t + 1}^f = F({\boldsymbol{x}}_t^a) $$ (24) 在各物理量符合收敛条件判定准则之后,将最后一次迭代的集合元素均值作为整个数据同化过程的来流条件修正值,代入FLUENT进行重新计算,得到修正后的流场分布。
2 实验过程和结果
RAE 2822是一个二维跨声速湍流流动的经典翼型,被许多国外的项目合作组(如EUROVAL)选作经典算例[21];NACA 0012作为经典的对称翼型,有实验数据可用来验证其数值模拟的准确性,故本文实验使用二维翼型RAE 2822和NACA 0012进行验证。RAE 2822翼型实验主要用来展现重构的收敛过程,ETKF对迎角、马赫数的影响,以及与其他方法在精度上的对比;NACA 0012翼型实验则面向风洞测量中基于有限测点重构的需求,用以探究影响ETKF重构精度的因素。
2.1 RAE 2822翼型压力重构
2.1.1 网格和计算条件设置
使用ICEM软件对RAE 2822进行网格划分(图2),采用四边形结构化网格,机翼模型弦长c = 1 m,计算域在弦长方向(即x方向)为40 m,弦长垂直方向(即y方向)为40 m,网格质量如表1所示。本文笛卡尔坐标系的原点位于机翼最前缘点。将FLUENT软件的计算模型设置为S–A湍流模型,此模型对跨声速流动的求解有较好的效果,且计算成本相对较低。自由流空气设置为可压缩空气,计算方式为稳态计算。实验数据来源为Cook等[22]的案例6中RAE 2822跨声速实验压力数据,其实验工况为:Ma = 0.725,α = 2.92°,Re = 6.2 × 106。共有103个测量点,其中一部分测点与机翼网格点难以匹配,会给数据同化带来较大误差,将其去除后剩余的压力测点个数为75(即$m = 75$)。
表 1 RAE 2822 网格质量及节点数量Table 1 Quality and node number of RAE 2822 mesh单元质量最小值 网格节点数量 第一层网格高度/m 值 0.9441 77429 4.2 × 10−6 2.1.2 RAE 2822翼型ETKF数据同化结果
2.1.2.1 迎角及马赫数
ETKF数据同化首先需要进行初始集合的构建,在一定抽样范围内进行拉丁超立方抽样,抽样范围根据经验进行合理选取。风洞对模型周围流场存在干扰,需要对给定的原始边界条件进行一定程度的修正。在Cook等的报告中建议的迎角修正值为2.54°,EUROVAL项目报告中采用线性理论方法对RAE 2822的案例6进行迎角及马赫数修正[21],修正的迎角为2.31°,马赫数为0.729,基于此修正值的CFD计算结果比原始值与实验数据吻合得更好,故以此线性理论修正值作为初始集合参考均值。在2.00~2.62范围内进行集合成员迎角的抽样取值,在0.700~0.758范围内进行马赫数的抽样取值,集合成员数量$k = 40$,初始抽样后的分布情况见图3、4深蓝色部分,40组集合成员初始的迎角均值为2.31°,马赫数均值为0.729。以此初始值进行40次FLUENT计算,由所有计算结果得到第一次迭代的先验分布矩阵${\boldsymbol{X}}_t^f$(矩阵维数:$309718 \times 40$),经ETKF迭代后得到后验分布矩阵${\boldsymbol{X}}_t^a$,通过式(23)计算${\boldsymbol{X}}_t^f$与${\boldsymbol{X}}_t^a$的压力系数、密度、x方向速度分量u和y方向速度分量v的均方误差。第一次迭代得到的后验分布矩阵${\boldsymbol{X}}_t^a$中迎角均值为2.308°,马赫数均值为0.7334(图5),将其作为第二次迭代的初始值再进行40次FLUENT计算,迭代过程如此进行,直至满足收敛判定条件。
图6为迭代过程中所有网格点的密度(ρ)、速度分量(u、v)和压力系数(Cp)的均方误差MSE变化曲线。从图中可以看出,在进行了第3次迭代之后,所有物理量的均方误差已经小于第一次迭代的1%,这表明在第3次迭代时ETKF就已经满足收敛要求,继续迭代发现均方误差可以进一步降低,于是迭代继续进行。
随着迭代的进行,集合中所有成员的边界条件都在产生变化并且更加集中(图3和4)。计算集合成员的方差如表2所示,可以看到在数据同化过程中,随机抽样产生的所有集合成员在向更为准确的修正值变化,且马赫数的集中效果要比迎角的集中效果更好。最终,集合成员迎角及马赫数均值在第10次迭代之后分别收敛为2.434°和0.7328(图5)。
表 2 ETKF前后集合成员迎角、马赫数的均值及方差Table 2 Mean and variance of ensemble angle of attack and Mach number before and after ETKF迎角 马赫数 初始均值 2.310° 0.729 ETKF后均值 2.434° 0.7328 初始方差 3.192 × 10−2 2.770 × 10−4 ETKF后方差 1.039 × 10−3 2.770 × 10−7 2.1.2.2 压力系数、升力系数和力矩系数
基于图2网格,将收敛后集合成员的迎角及马赫数均值(表3)作为边界条件代入FLUENT进行重新计算,得到ETKF同化后的压力系数Cp曲线分布,此结果即为ETKF数据同化的最终结果。将ETKF同化结果与线性理论修正值进行对比,结果如图7和8所示。
表 3 RAE 2822 case 6边界条件Table 3 Boundary condition of RAE 2822 case 6迎角/(°) 马赫数 雷诺数 原始条件 2.92 0.725 6.5 × 106 线性理论 2.31 0.729 6.5 × 106 ETKF 2.43 0.733 6.5 × 106 从图7和8中可以看出:与线性理论修正后的计算结果相比,ETKF同化后的压力系数曲线更加靠近实验数据;线性理论的迎角修正值过大,导致其计算误差与ETKF相比更大,尤其是在激波位置。从数值上分析(式(25))可得,ETKF同化的压力系数平均相对误差e比线性理论修正后降低了约3%。e的计算方法如下:
$$ e = \frac{1}{j}\sum\limits_{i\;=\;1}^j {\left| {\frac{{C{_{p,\;\exp }} - C_p}}{{C{_{p,\;\exp }}}}} \right|} $$ (25) 式中:j为实验测点的数量,$C{_{p,\;\exp }}$为实验测点的压力系数。
将ETKF同化后的升力系数$C_L$及力矩系数$C_m$与线性理论修正值一同与实验测量值进行对比(表4)。从数据上可以看出,使用ETKF数据同化得到的升力系数及力矩系数比线性理论修正值更符合实验测量的情况(其与实验值的误差均小于1%)。
表 4 ETKF同化和线性理论修正后CL和Cm、激波位置Cp平均相对误差与实验值的对比Table 4 Comparison of CL, Cm and Cp average relative error near the shock wave position between ETKF, linear theory and experimentCp平均相对误差 CL CL误差 Cm Cm误差 实验值 — 0.7430 — −0.0950 — ETKF 5.741% 0.7379 0.67% −0.0941 0.95% 线性理论 8.881% 0.7120 4.17% −0.0918 3.37% 2.2 NACA 0012翼型压力重构
2.2.1 网格和计算条件设置
如图9和表5所示,NACA 0012翼型采用的计算网格为四边形结构化网格,机翼模型弦长c =0.1524 m,计算域在弦长方向为4.5 m,在垂直弦长方向为5 m,网格质量高,FLUENT计算时选用S–A湍流模型。实验数据来源于NASA进行的NACA 0012翼型风洞实验[23],选取TEST 119的压力数据进行数据同化的测试,其实验工况为:Ma = 0.4022,α = 4.0208°,Re = 6.0706 × 106。共有46个压力测量点,由于靠近机翼前缘位置的数据在同化时难以与网格节点进行匹配,容易带来较大误差,故在同化时将其去除,仅使用其中44个数据(即$m \leqslant 44$)。
表 5 NACA0012 网格质量及节点数量Table 5 Quality and node number of NACA 0012 mesh最小单元 节点数量 第一层网格高度/m 值 0.8818 32050 1.0 × 10−5 NACA 0012翼型实验主要面向风洞测量中基于有限测点重构的应用需求,对比分析基于少数测点进行压力稀疏重构的可行性。图10给出了实验压力测点的位置及序号。实验共进行4组,其中3组实验分别选用上翼面(序号1~22)的不同位置6个实验测点进行数据同化,第4组实验作为同化结果的对标,使用所有数据测点进行同化处理。
2.2.2 NACA 0012翼型ETKF数据同化结果
在数据同化之前进行初始集合的抽样,因为此实验无具体参考修正值,故基于实验原始工况进行初始值的抽样,并设定较大的抽样范围。设置集合成员数量k = 40,在3.52~4.52范围进行迎角抽样,在0.3722~0.4322范围进行马赫数抽样,得到初始集合的迎角均值为4.0208°,马赫数均值为0.4021。采用第1节的理论与流程进行数据同化,同化实验1~4均从此初始值开始分别进行迭代,以对比使用不同实验测量数据得到的迭代效果。4组实验所用的压力测点及迭代至收敛所需的迭代次数见表6。
表 6 4组实验的压力测点序号及迭代次数Table 6 The number of pressure measuring points and iteration times of 4 groups of experiments使用的压力测点序号 迭代次数 同化实验1 1,2,3,4,5,6 3 同化实验2 7,8,9,10,11,12 3 同化实验3 1,5,9,13,17,21 3 同化实验4 1~44(所有测点) 4 实验结果如表7和图11所示,表7中压力系数平均相对误差由式(25)计算得到,4组同化实验均使用上翼面实验测量值进行误差的计算(即j = 22)。如图11所示,相比于深绿色点划线表示的未同化实验数据,经过ETKF同化的压力系数均有一定的优化效果。同化实验4使用了所有实验测量点进行同化,其结果的误差最小,只有1.05%,说明ETKF方法的同化精度与使用的数据量有关,测点数据越多,得到的同化效果越好;只采用6个测点进行同化的实验1~3也达到了小于6%的平均相对误差,证实了基于有限测点进行压力稀疏重构、实现全域感知的可行性。且对比同化实验1~3的结果可以发现,重构精度与初始测点位置的选取密切相关,初始测点位置不同,相对误差从2.42%到5.74%不尽相同,这说明在风洞实验时可以基于重构算法进行传感测点位置的优化布置。
表 7 4组实验ETKF后的迎角、马赫数及压力系数平均相对误差Table 7 Angle of attack, Mach number and pressure coefficient average relative error after ETKF in 4 groups of experiments迎角/(°) 马赫数 压力系数平均相对误差 同化实验1 3.3932 0.3884 2.42% 同化实验2 3.7286 0.3937 5.74% 同化实验3 3.5340 0.3928 3.77% 同化实验4 3.4159 0.3131 1.05% 未同化实验 4.0208 0.4022 8.72% 3 结 论
本文针对风洞测量中有限测点进行气动压力重构开展研究,使用集合变换卡尔曼滤波方法对二维翼型RAE 2822和NACA 0012的湍流流场进行数据同化,结合稀疏的实验测量压力数据,对两种翼型的实验迎角及马赫数进行修正,通过修正后的边界条件进行重新计算得到了全域流场。将ETKF同化后的机翼表面压力系数及机翼的升力系数、力矩系数与修正前数据和线性理论修正值作对比,得到以下结论:
1) ETKF可以通过有限的测量信息进行流场的全域重构,其重构的精度与测量点的选取有直接关系,在实验测量位置进行压力系数的修正,其精度比经典线性理论更加靠近实验测量值。
2) 使用ETKF修正后的迎角及马赫数计算得到的机翼升力系数及力矩系数与实验测量值之间的误差很小,这表明了基于ETKF数据同化的有效性。
3) 由于ETKF进行同化的数据基础是实验测量数据,其对于流场的估计精度取决于此测量值的准确性。
本文研究表明,作为一种用于湍流流场的数据同化方法,集合变换卡尔曼滤波能够有效利用CFD数值计算数据和EFD高精度稀疏测量数据,重构得到更高精度的全域流场,适用于湍流场稳态计算的分布预测。本文在给定了实验迎角及马赫数的条件下进行了压力场稀疏重构,后续可以进一步进行其他分布条件下(如剪应力分布)的数据同化实验,进行多数据融合下的流场重构,推进风洞实验测量技术向高效化发展。
-
-
[1] 国义军, 代光月, 桂业伟, 等.碳基材料氧化烧蚀的双平台理论和反应控制机理[J].空气动力学学报, 2014, 32(6):755-760. DOI: 10.7638/kqdlxxb-2014.0047 Guo Y J, Dai G Y, Gui Y W, et al. A dual platform theory for carbon based material oxidation with reaction diffusion rate controlled kinetics[J]. Acta Aerodynamica Sinica, 2014, 32(6):755-760. DOI: 10.7638/kqdlxxb-2014.0047
[2] Scala S M. The ablation of graphite in dissociated air, Part Ⅰ: Theory[R]. AD289298, 1962.
[3] Diaconis N S, Gorsuch P D, Sheridan R A. The ablation of graphite in dissociated air, Part Ⅱ: Experimental investigation[R]. AD290051, 1962.
[4] Maahs H G. Oxidation of carbon at high temperatures: reaction-rate control or transport control[R]. NASA TN D-6310, 1971.
[5] Swann R T, Pittman C M, Smith J C. One-dimensional numerical analysis of the transient response of thermal protection systems[R]. NASA TN D-2976, 1965.
[6] 黄振中.烧蚀端头的瞬变外形及内部温度分布[J].空气动力学学报, 1981(1):53-65. http://www.cnki.com.cn/Article/CJFDTOTAL-KQDX198101005.htm [7] 卞荫贵, 钟家康.高温边界层传热[M].北京:科学出版社, 1986. [8] 国义军.炭化材料烧蚀防热的理论分析与工程应用[J].空气动力学学报, 1994, 12(1):94-99. http://www.cnki.com.cn/Article/CJFDTOTAL-KQDX401.014.htm Guo Y J. An analysis of a charring ablative thermal protection system with its engineering application[J]. Acta Aerodynamica Sinica, 1994, 12(1):94-99. http://www.cnki.com.cn/Article/CJFDTOTAL-KQDX401.014.htm
[9] Milos F S, Chen Y K. Comprehensive model for multicomponent ablation thermochemistry[R]. AIAA-97-0141, 1997.
[10] 黄海明, 杜善义, 吴林志, 等. C/C复合材料烧蚀性能分析[J].复合材料学报, 2001, 18(3):76-80. DOI: 10.3321/j.issn:1000-3851.2001.03.018 Huang H M, Du S Y, Wu L Z, et al. Analysis of the ablation of C/C composites[J]. Acta Materiae Compositae Sinica, 2001, 18(3):76-80. DOI: 10.3321/j.issn:1000-3851.2001.03.018
[11] 张志成, 潘梅林, 刘初平.高超声速气动热和热防护[M].北京:国防工业出版社, 2003. [12] Welsh W E, Chung P M. A modified theory for the effect of surface temperature on the combustion rate of carbon surface in air[C]//Proc of Heat Transfer and Fluid Mechanics Institute.: Stanford University Press, 1963: 146-159.
[13] Baron J R, Bernstein H. Heterogeneous rate coupling for graphite oxidation[J]. AIAA Journal, 1971, 9(8):1588-1595. DOI: 10.2514/3.49961
[14] Milos F S. Comparison of ablation predictions for carbonaceous materials using CEA and JANAF-based species thermodynamics[C]. Proc of the 35th Annual Conference on Composites, Materials, and Structures. 2011.
[15] 益小苏, 杜善义, 张立同.复合材料手册[M].北京:化学工业出版社, 2009. [16] Clark R K. An analysis of a charring ablator with thermal nonequilibrium, chemical kinetics, and mass transfer[R]. NASA TN D-7180, 1973.
-
期刊类型引用(1)
1. 孟宇,顾洪斌,张新宇. 微波对超声速燃烧火焰结构的影响. 航空学报. 2019(12): 88-96 .  百度学术
百度学术
其他类型引用(6)







 下载:
下载: